Buffers at Work
Controlling pH · Project 2
Abdur-Rahman Bilal
Lead Data Analyst, Slide Designer
Abdullah Saleh
Lead Researcher
Adam Bourraman
Concept and Draft Reviewer
Project Overview
“How do buffer solutions minimize changes in pH, and why are buffers important in chemical and biological systems?”
What This Project Includes
- ●Clear definitions of pH, acids, bases, and buffers
- ●Analysis of acetic acid/acetate buffer system
- ●Data visualization comparing buffered vs unbuffered solutions
- ●Real-life applications in biology, digestion, and medicine
Key Idea: Buffers maintain stable pH because a weak acid and its conjugate base can neutralize small amounts of added acid or base before significant pH changes occur.
Understanding pH
pH measures the acidity or basicity of a solution and is related to the concentration of hydronium ions (H3O+).
pH = −log10[H3O+]
At 25°C:
pH < 7
Acidic
pH = 7
Neutral (pure water)
pH > 7
Basic (alkaline)
Important: The pH scale is logarithmic! A 1-unit change means a 10x change in [H3O+]. Small acid or base additions can cause dramatic pH shifts without a buffer.
Acids, Bases, and Conjugate Pairs
Acid
Donates a proton (H+)
Base
Accepts a proton (H+)
A conjugate pair differs by exactly one H+:
Acetic Acid / Acetate
CH3COOH ⇌ CH3COO− + H+
Ammonia / Ammonium
NH3 + H+ ⇌ NH4+
How Conjugate Pairs Create Buffers
In a buffer solution, both members of a conjugate pair are present. When acid (H+) is added, the conjugate base removes it. When base (OH−) is added, the weak acid removes it. This dual action keeps pH stable!
What Is a Buffer Solution?
A buffer is a special mixture that resists big changes in pH when small amounts of acid or base are added. Buffers are made from:
- A weak acid + its conjugate base, OR
- A weak base + its conjugate acid
The result: pH stays stable instead of swinging wildly.
If Acid (H+) Is Added
The conjugate base removes it:
A− + H+ → HA
If Base (OH−) Is Added
The weak acid removes it:
HA + OH− → A− + H2O
Why Buffers Matter
Many chemical reactions and biological processes only work within narrow pH ranges. Without buffers, enzymes stop working and reactions fail. Buffers maintain stable conditions so life and chemistry can function normally.
Our Buffer System: Acetic Acid / Acetate
CH3COOH ⇌ CH3COO− + H+
This equilibrium allows the buffer to neutralize both acids and bases. When H+ is added, acetate (CH3COO−) removes it. When OH− is added, acetic acid (CH3COOH) removes it.
pKa
4.76
Optimal pH Range
3.76 to 5.76
Our Experiment pH
~4.7 to ~4.8
- ●Acetic acid/acetate is a common laboratory buffer with well-documented behavior
- ●The pKa of 4.76 makes it ideal for maintaining pH in the acidic range
- ●This buffer system is used in many biochemical applications and food preservation
- ●The same buffering principles apply to all buffer systems, including the bicarbonate buffer in blood
Our Experimental Approach
Hypothesis: A buffered solution (CH3COOH/CH3COO−) will resist pH changes much better than an unbuffered solution when the same amount of acid or base is added.
Contains CH3COOH (acetic acid)
Contains CH3COO− (acetate ion)
Initial pH: ~4.77
Can neutralize added H+ or OH−
Pure water (distilled)
No weak acid/base pair
Initial pH: ~7.06
Cannot neutralize additions
Dataset: Acid Addition Experiment
We measured the effect of adding equal amounts of strong acid (HCl) to both buffered and unbuffered solutions, recording pH after each addition.
| Trial | Buffered pH | Unbuffered pH | Difference |
|---|---|---|---|
| 0 (baseline) | 4.80 | 7.05 | 2.25 |
| 1 | 4.76 | 3.33 | 1.43 |
| 2 | 4.70 | 3.03 | 1.67 |
| 3 | 4.68 | 2.94 | 1.74 |
| 4 | 4.70 | 2.68 | 2.02 |
| 5 | 4.67 | 2.64 | 2.03 |
| 6 | 4.59 | 2.38 | 2.21 |
| 7 | 4.58 | 2.46 | 2.12 |
| 8 (final) | 4.58 | 2.39 | 2.19 |
Buffered Total Change
0 pH
Unbuffered Total Change
0 pH
Unbuffered Changed
0x more!
Ratio: 4.66 / 0.22 ≈ 21.18
Interactive Trial Explorer
The Unbuffered Solution Changed
more than the buffered solution
Graphs
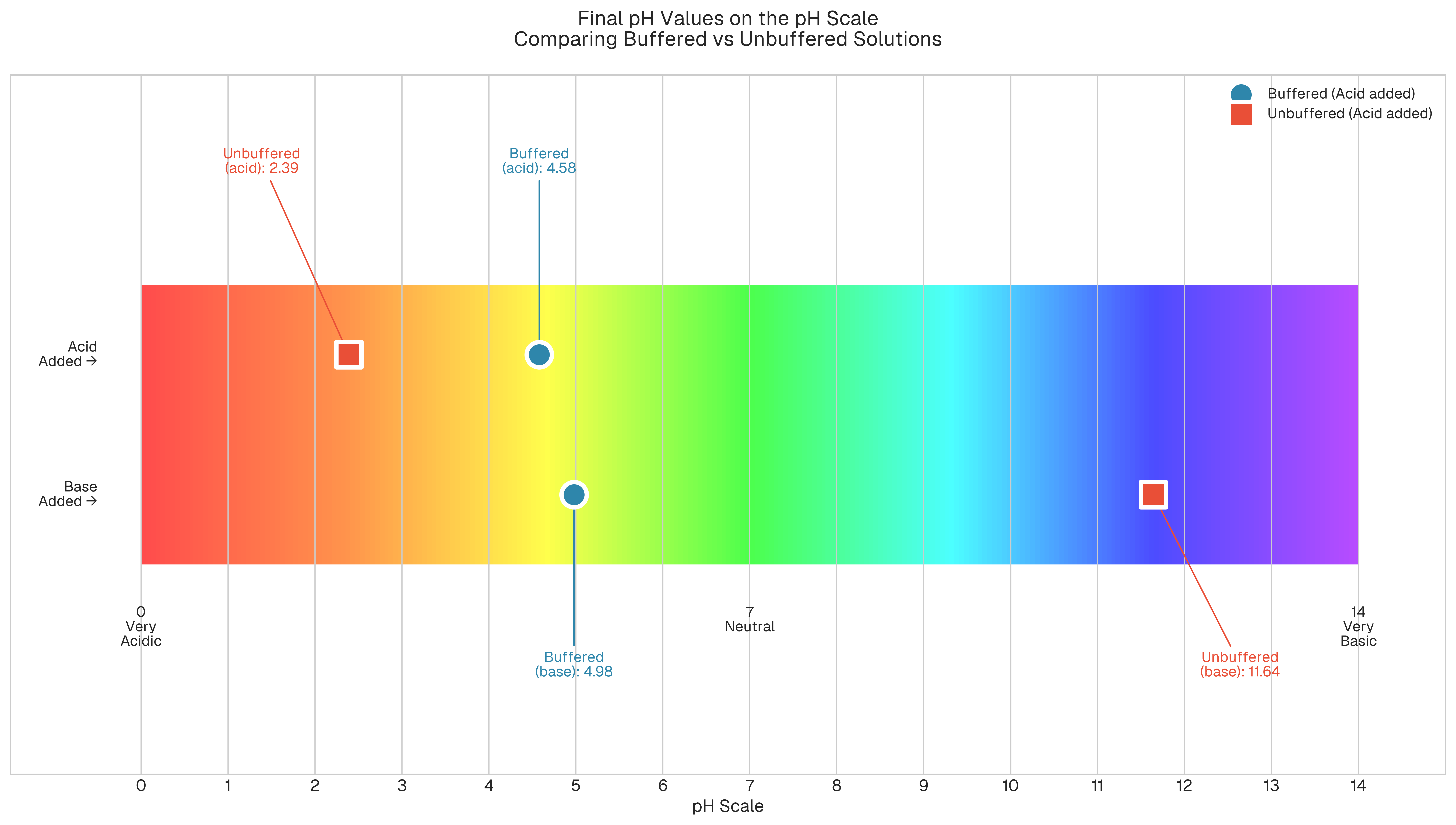
What this shows: The buffered solution stayed within a narrow acidic range (4.58 to 4.80), while the unbuffered solution swung from neutral (7.05) to strongly acidic (2.39).
Our Claim, Evidence, and Reasoning
Buffer solutions resist pH changes significantly better than unbuffered solutions when exposed to the same amounts of acid or base through a chemical balancing act.
After 8 additions of acid: the buffered solution changed from pH 4.80 to 4.58 (ΔpH ≈ 0.22), while the unbuffered solution changed from pH 7.05 to 2.39 (ΔpH ≈ 4.66). The unbuffered solution experienced approximately 21 times more pH change.
Buffers work through neutralizing reactions. When H+ is added, the conjugate base (acetate) reacts with it to form the weak acid, removing H+ from solution and preventing pH drops.
When OH− is added, the weak acid (acetic acid) donates H+ to neutralize it, forming water and preventing pH rises.
This dual mechanism means free H+ concentration changes minimally. Unbuffered solutions lack these chemical defenses, so pH swings dramatically with each addition. The buffer absorbs pH changes instead of letting them affect the solution.
Buffers in Real Life
Blood uses the bicarbonate buffer (H2CO3/HCO3−) to maintain pH at 7.40. This buffer works on the same principle as our acetate buffer, but operates in a different pH range. The buffering mechanism is identical: neutralizing acids and bases to maintain stability.
Acetic acid (vinegar) is used as a preservative and pH buffer in pickling and food processing. The acetate buffer system we studied maintains stable acidic conditions that inhibit bacterial growth while preserving food quality.
Acetate buffers are widely used in biochemistry and molecular biology labs to maintain stable pH during experiments. Many enzymes and proteins require specific pH conditions to function, making buffers essential for research.
The Math Behind Buffers
pH = pKa + log10([A−] / [HA])
This equation predicts buffer pH based on the ratio of conjugate base [A−] to weak acid [HA]:
pH
The pH of the buffer solution
pKa
The negative log of the acid dissociation constant (unique to each weak acid)
[A−]
Concentration of the conjugate base (e.g., CH3COO− acetate)
[HA]
Concentration of the weak acid (e.g., CH3COOH acetic acid)
Key Insight: When [A−] = [HA], the log term becomes 0, so pH = pKa. This is where buffers work best!
Buffer Capacity and Limitations
Buffer capacity is the amount of acid or base a buffer can neutralize before its pH changes significantly. Higher concentrations of buffer components = greater capacity to resist pH change.
- ●Concentration: More buffer = more capacity
- ●Ratio: Works best when [A−] ≈ [HA] (1:1)
- ●pH range: Most effective within ±1 pH unit of pKa
- ●Can be overwhelmed by large acid/base additions
- ●Only effective in a narrow pH range
- ●Temperature can affect buffer performance
Our acetate buffer successfully neutralized 8 acid additions while maintaining pH between 4.58 and 4.80. This demonstrates excellent buffer capacity within its effective range. The unbuffered solution, lacking this capacity, experienced dramatic pH changes from 7.05 down to 2.39.
Conclusions and Key Takeaways
- ✓Buffer solutions maintain stable pH through the presence of a weak acid and its conjugate base
- ✓Our data showed buffered solutions experienced 21x less pH change than unbuffered solutions
- ✓The acetic acid-acetate buffer we studied works on the same principles as biological buffers like bicarbonate in blood
- ✓Buffers have wide-ranging applications in biology, food science, medicine, and chemistry
Understanding buffers helps us appreciate how chemical systems maintain the precise pH conditions needed for countless processes, from preserving food to keeping us alive.
- →Test different buffer systems (phosphate, citrate) to compare effectiveness at various pH ranges
- →Investigate how temperature affects buffer capacity
- →Explore buffer failure points and saturation limits experimentally
AI Usage Disclosure
We used Claude AI to redesign and enhance our original presentation slides, expand content, integrate our graphs with proper formatting, and develop speaker scripts. We also used Claude Opus 4 to develop and animate our project website.
All experimental design, data generation, graph creation, original slides, original website content, research, and scientific analysis were completed by our team.
© 2025 Abdur-Rahman Bilal, Abdullah Saleh, Adam Bourraman. All rights reserved.
References and Sources
- OpenStax Chemistry 2e, Chapter 14: Acid-Base Equilibria
- Chemistry LibreTexts: Buffers, Solutions That Resist pH Change
- Pearson Education: Buffers Explained, Definition and Examples
- Wikipedia: Buffer Solution
- Project 2 Guide and Student Checklist (Class handouts)
All graphs and data were generated for this project using Python with matplotlib. Acid dataset is listed above. Base dataset is a simulated dataset created for visualization.
View full references page →